Finding FDA authorized devices with Predetermined Change Control Plans
AI/ML
datasets
FDA
medical devices
Summary
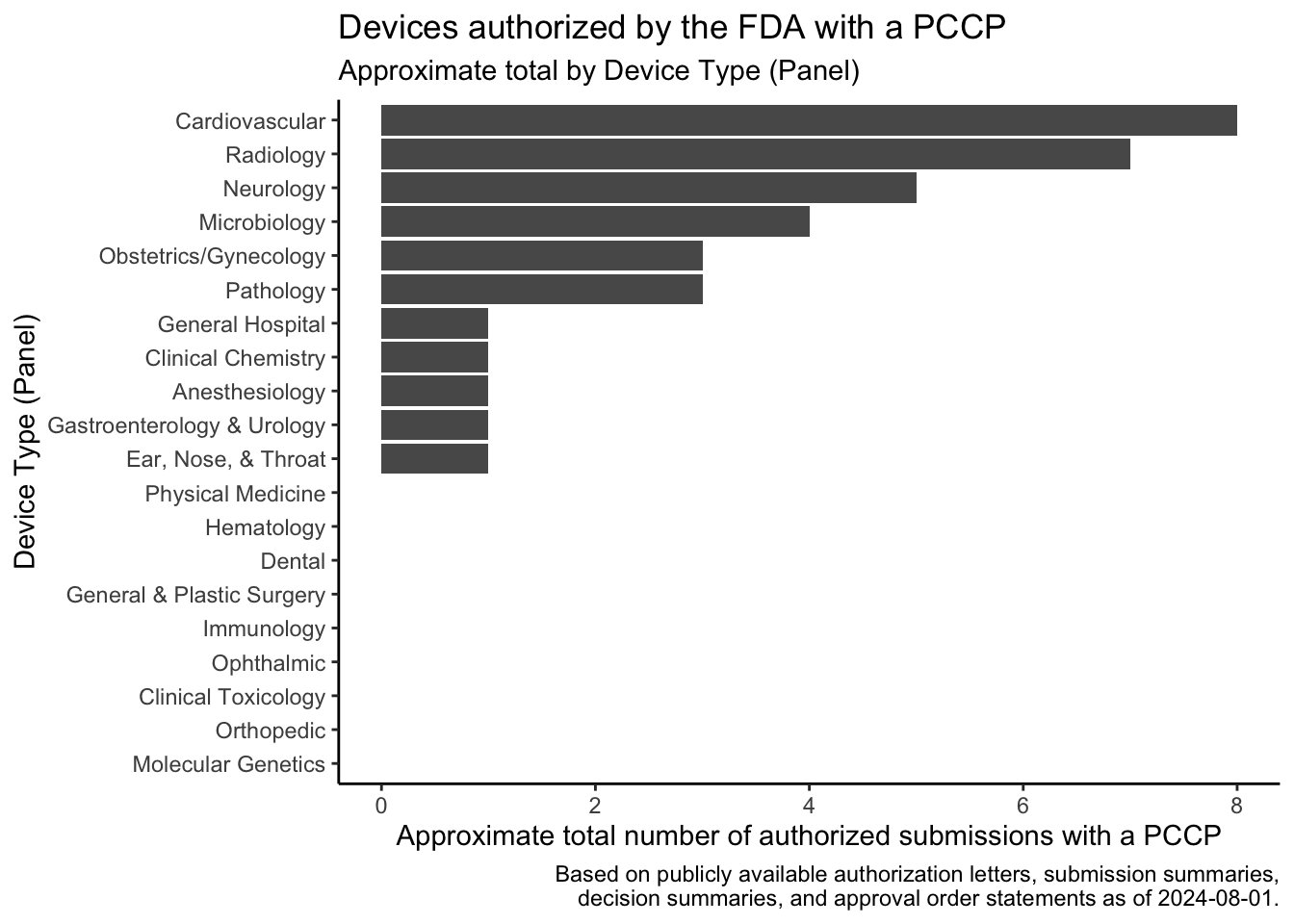
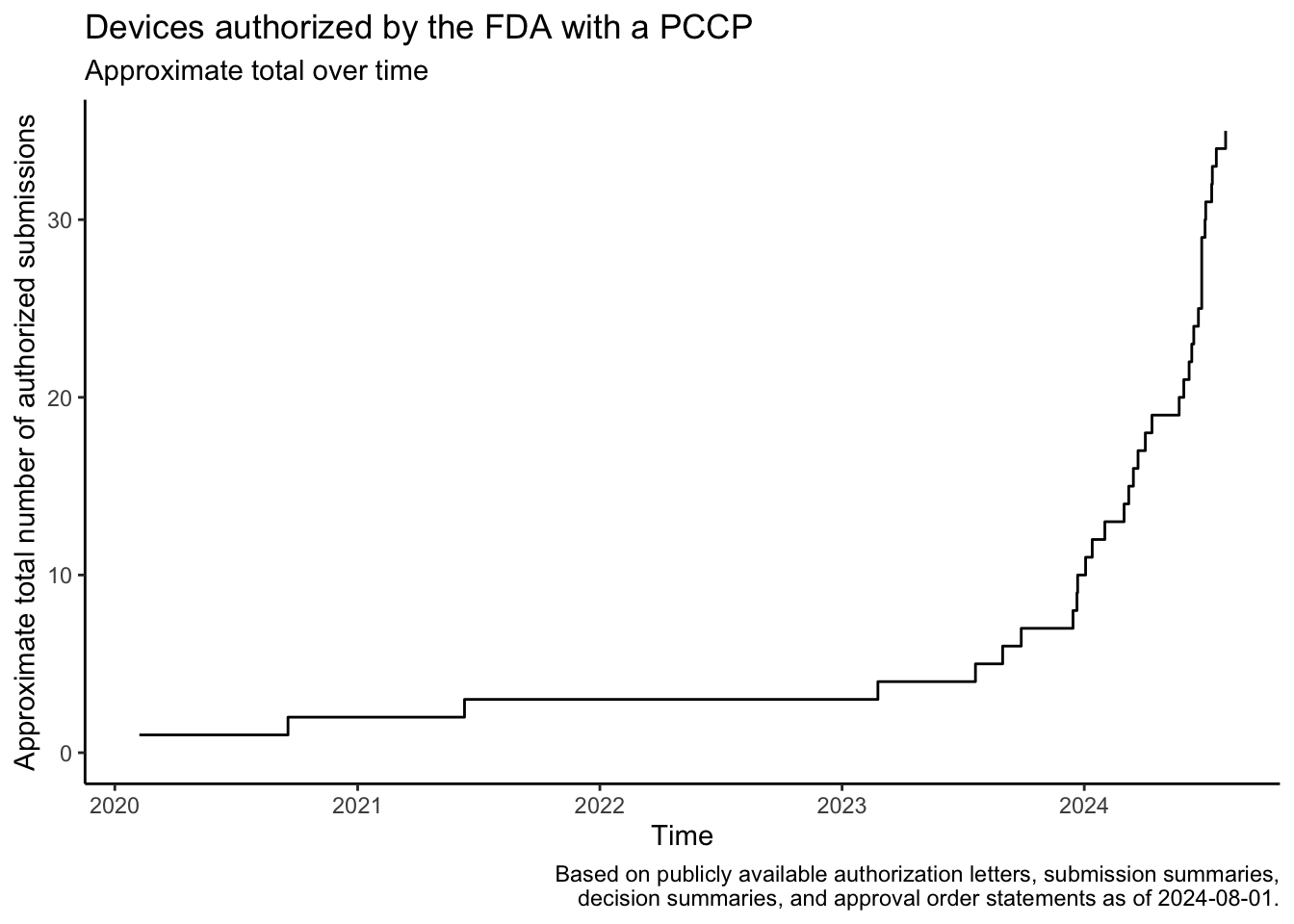
In December 2022, the Food and Drug Omnibus Reform Act of 2022 provided the FDA with new authority related to the authorization of Predetermined Change Control Plans (PCCPs). A PCCP can enable significant planned modifications to be made to a medical device after the device and the plans to modify the device have been reviewed by the FDA. This post provides a summary of the law, describes how to find devices that have been authorized with PCCPs, lists devices that I found using these approaches, shows the approximate rate at which the FDA has authorized these devices over time based on the search results, and shows the device areas where PCCPs are most commonly used based on the search results.
Background
Note
The information in this post is based on sources in the public domain. It is provided here “as-is” without warranty of any kind. For reliable and up-to-date information, refer to the FDA’s website.
Predetermined Change Control Plans (PCCPs) can enable significant modifications to be made to medical devices that have been reviewed by the FDA. When these modifications are made in accordance with a PCCP that the FDA has authorized, the modifications themselves do not require additional review by the FDA.
In December 2022, the Food and Drug Omnibus Reform Act of 2022 provided the FDA with new authority related to the authorization of PCCPs for medical devices.1 PCCPs are now described in section 515C of the Food, Drug, and Cosmetic Act (FD&C Act). Paraphrased and simplified, this says:
As a part of a premarket submission, the FDA may authorize PCCPs for “planned changes that may be made to the device” as long as “the device remains safe and effective without any such change” and, for 510(k) devices, “the device would remain substantially equivalent to the predicate.”2
Section 515C of the FD&C Act also describes what the FDA may require a PCCP to include.3 The FDA may require that a PCCP include information about:
Labeling required for safe and effective use as the device changes,
Notification requirements if the device does not function as intended, and
Performance requirements for changes made under the plan.
In addition, in April 2023, the FDA issued a draft guidance with proposed recommendations related to PCCPs for artificial intelligence and machine learning enabled medical devices.4 [1]
NoteUpdates
On 2024-12-03, the FDA revised and finalized its guidance on PCCPs for artificial intelligence and machine learning enabled medical devices. [2]
On 2024-08-21, the FDA published a new draft guidance on PCCPs for any device (not just AI or ML related devices) on its website: Predetermined Change Control Plans for Medical Devices. [3]
Even when a device developer does not plan to establish a PCCP for a medical device under development, the existence of a PCCP for a predicate device can impact the regulatory strategy for a device that will go through the 510(k) pathway. The statute says:
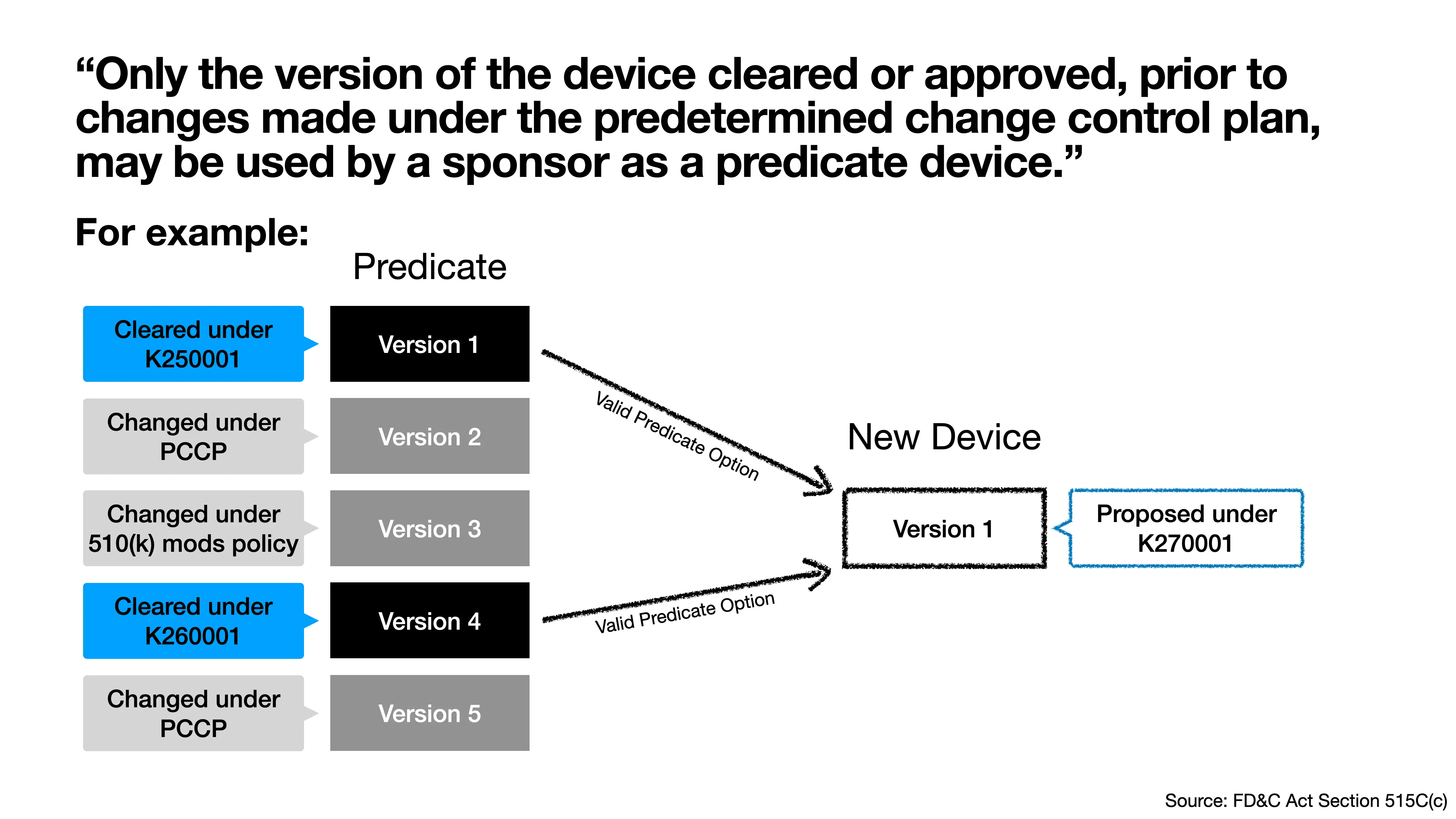
Only the version of the device cleared or approved, prior to changes made under the predetermined change control plan, may be used by a sponsor as a predicate device.5
See the example in Figure 1, which shows a potential predicate device that has been cleared by the FDA with a PCCP (Version 1), modified under the PCCP (Version 2), modified again without additional FDA review under the 510(k) modifications policy [4] (Version 3), cleared again by the FDA (Version 4), and then modified again under the PCCP (Version 5). If a device developer wants to use this device as a predicate device, the developer should compare the new device to either Version 1 or Version 4 because the other versions are not a “version of the device cleared or approved, prior to changes made under the predetermined change control plan”.
It is important for a device developer to know whether a device they are considering using as a predicate has a PCCP.
Finding devices with PCCPs using a search engine
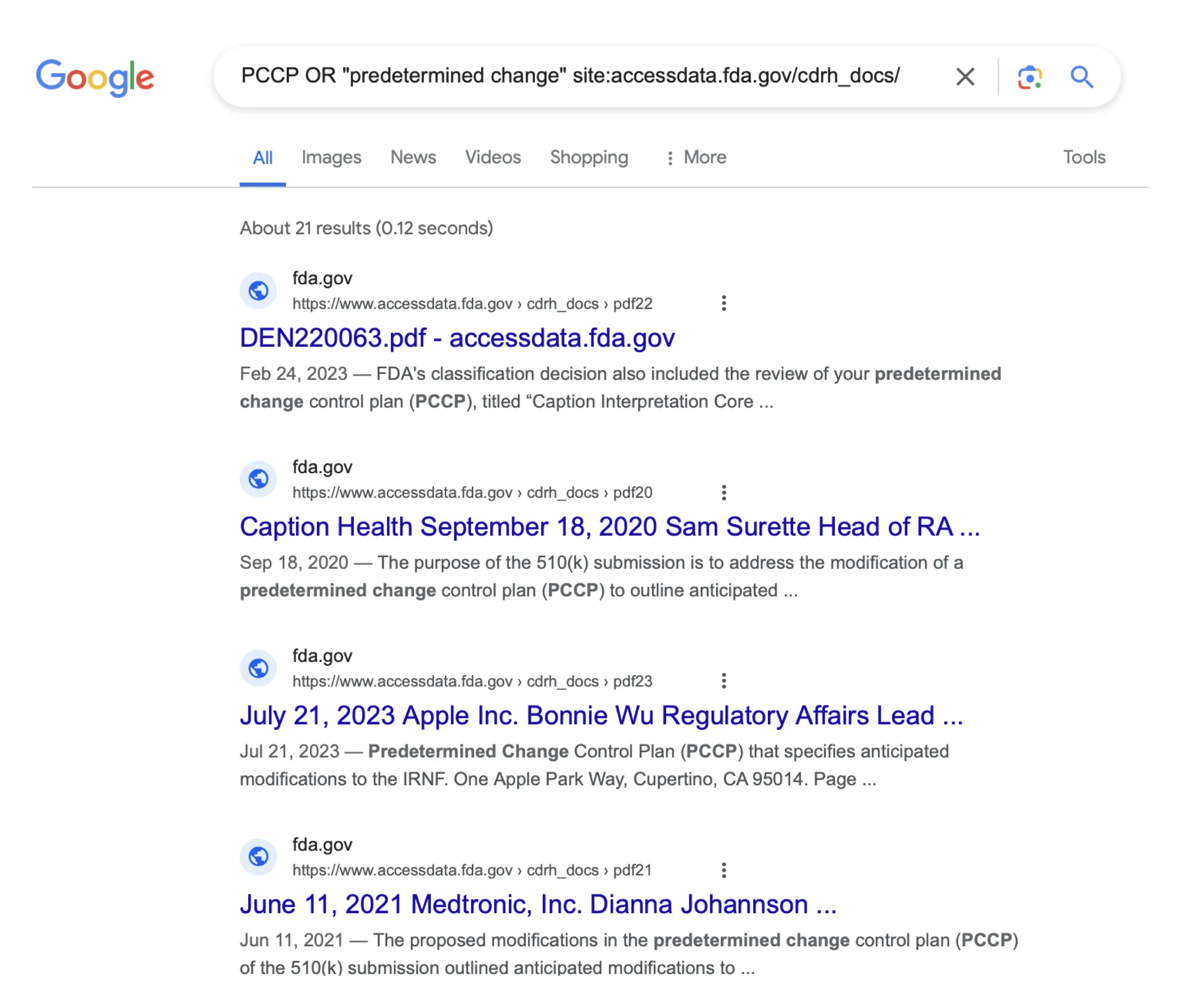
One way to identify devices that may include a PCCP is through a site-specific search engine query. An example, showing results from Google as of 2024-04-19, is shown here6:
This shows that there were 21 documents that included either the term “PCCP” or the term “predetermined change” on the site where the FDA posts submission and review summaries as of 2024-04-19. Some submissions returned multiple documents because, for example, both a submission summary document and a review summary document may be available for De Novos and for in vitro diagnostic 510(k)s. In addition, some of the results may be included because a 510(k) summary discusses a PCCP for a predicate device even if the device that was the subject of the 510(k) did not include a PCCP.
The search results show that the FDA authorized a few devices with PCCPs before the law was passed in December 2022. Several of these devices came to market through the De Novo program or in regulations recently established through the De Novo program, and the De Novo authorizations associated with these devices typically also established special controls related to PCCPs.
The results show that the FDA is including additional language in clearance letters when there is a PCCP. Specifically, many of these letters include the following paragraph:
FDA’s substantial equivalence determination also included the review and clearance of your Predetermined Change Control Plan (PCCP). Under section 515C(b)(1) of the Act, a new premarket notification is not required for a change to a device cleared under section 510(k) of the Act, if such change is consistent with an established PCCP granted pursuant to section 515C(b)(2) of the Act. Under 21 CFR 807.81(a)(3), a new premarket notification is required if there is a major change or modification in the intended use of a device, or if there is a change or modification in a device that could significantly affect the safety or effectiveness of the device, e.g., a significant change or modification in design, material, chemical composition, energy source, or manufacturing process. Accordingly, if deviations from the established PCCP result in a major change or modification in the intended use of the device, or result in a change or modification in the device that could significantly affect the safety or effectiveness of the device, then a new premarket notification would be required consistent with section 515C(b)(1) of the Act and 21 CFR 807.81(a)(3). Failure to submit such a premarket submission would constitute adulteration and misbranding under sections 501(f)(1)(B) and 502(o) of the Act, respectively. Additional information about changes that may require a new premarket notification are provided in the FDA guidance documents entitled “Deciding When to Submit a 510(k) for a Change to an Existing Device” (https://www.fda.gov/media/99812/download) and “Deciding When to Submit a 510(k) for a Software Change to an Existing Device” (https://www.fda.gov/media/99785/download).
This language is informative, and it can also help us find devices that have PCCPs. If the FDA did not include this language in its letters, we would need to rely on the 510(k) summary – which is written by the device developer and is not available for all devices – to determine whether a 510(k) clearance included a PCCP. Still, even though this language is appearing in letters from the FDA, we should not assume perfection: There may be cases where the FDA has included this language in a clearance letter when a PCCP was not part of the submission, and there may also be cases where the FDA did not include this language in a clearance letter when a PCCP was part of the clearance.7
The FDA also appears to be noting the presence of PCCPs in approval order statements for PMAs.8
Devices found using this search
Note
This table shows search results as of about 2024-08-01. Updates will be posted here.
References
[1]
“Marketing submission recommendations for a predetermined change control plan for artificial intelligence/machine learning (AI/ML)-enabled device software functions,” U.S. Food and Drug Administration, Draft Guidance Document. Docket Number FDA-2022-D-2628, Apr. 2023. Available: https://www.regulations.gov/document/FDA-2022-D-2628-0002
[2]
“Marketing submission recommendations for a predetermined change control plan for artificial intelligence-enabled device software functions,” U.S. Food and Drug Administration, Final Guidance Document. Docket Number FDA-2022-D-2628, Dec. 2024. Available: https://www.regulations.gov/document/FDA-2022-D-2628-0036
[3]
“Predetermined Change Control Plans for medical devices,” U.S. Food and Drug Administration, Draft Guidance Document. Docket Number FDA-2024-D-2338, Aug. 2024. Available: https://www.regulations.gov/document/FDA-2024-D-2338-0002
[4]
“Deciding when to submit a 510(k) for a change to an existing device,” U.S. Food and Drug Administration, Final Guidance Document. Docket Number FDA-2011-D-0453, Oct. 2017. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/deciding-when-submit-510k-change-existing-device
[5]
“FDA fact sheet: CDRH’s approach to tumor profiling next generation sequencing tests,” U.S. Food and Drug Administration, Fact Sheet, Nov. 2017. Available: https://www.fda.gov/files/medical%20devices/published/CDRH’s-Approach-to-Tumor-Profiling-Next-Generation-Sequencing-Tests.pdf
Footnotes
See section 3308 of the Food and Drug Omnibus Reform Act of 2022, Title III of Division FF of the Consolidated Appropriations Act, 2023, Pub. L. No. 117-328 (“FDORA”), enacted on December 29, 2022 and available at: https://www.congress.gov/117/plaws/publ328/PLAW-117publ328.pdf, pp. 5835-5836.↩︎
See: FD&C Act Section 515C(a)(2) and (b)(2).↩︎
See: FD&C Act Section 515C(a)(3) and (b)(3).↩︎
See: https://www.fda.gov/medical-devices/medical-devices-news-and-events/cdrh-issues-draft-guidance-predetermined-change-control-plans-artificial-intelligencemachine.↩︎
See: FD&C Act Section 515C(c).↩︎
See: https://www.google.com/search?q=PCCP+OR+%22predetermined+change%22+site%3Aaccessdata.fda.gov%2Fcdrh_docs%2F.↩︎
I shared a draft of this post with the FDA before publishing it, and the FDA subsequently issued corrections for some of the clearance letters that had been posted on the FDA’s website. These corrections are accounted for in the results provided here.↩︎
See for example, P190016/S007 on 2024-02-01 and P160026/S040 on 2024-04-12.↩︎
To search the approval order statements, I used my
fdadatapackage for the R programming language: https://github.com/bjoleary/fdadata.↩︎See: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices.↩︎
Reuse
Citation
BibTeX citation:
@online{o'leary2024,
author = {O’Leary, Brendan},
title = {Finding {FDA} Authorized Devices with {Predetermined}
{Change} {Control} {Plans}},
date = {2024-08-20},
url = {https://www.boleary.com/blog/posts/202408-pccp/},
langid = {en},
abstract = {In December 2022, the Food and Drug Omnibus Reform Act of
2022 provided the FDA with new authority related to the
authorization of Predetermined Change Control Plans (PCCPs). A PCCP
can enable significant planned modifications to be made to a medical
device after the device and the plans to modify the device have been
reviewed by the FDA. This post provides a summary of the law,
describes how to find devices that have been authorized with PCCPs,
lists devices that I found using these approaches, shows the
approximate rate at which the FDA has authorized these devices over
time based on the search results, and shows the device areas where
PCCPs are most commonly used based on the search results.}
}
For attribution, please cite this work as:
B.
O’Leary, “Finding FDA authorized devices with Predetermined Change
Control Plans,” Aug. 20, 2024. https://www.boleary.com/blog/posts/202408-pccp/